Direct conversion of anhydrides to obtain high value-added lactones is currently a research hotspot concerning its high efficiency and energy-saving. But it is a great challenge to selectively hydrogenate the C = O bond in anhydride without side reactions or corrosion/poisoning of active metals in acidic system. Recently, Professor Liang Changhai and his group developed a kind of acid-resistant cobalt-nickel bimetallic silicide catalyst with noble metal-like properties for the selective hydrogenation of phthalic anhydride to phthalide. Very good activity and long-term stability in acidic environment were achieved with controllably selection of target products during the hydrogenation reaction. Under mild conditions, acid anhydride can be converted efficiently and selectively. Related work was published on the Catalysis Science & Technology (2019, 9, 1108-1116) and was selected as the front cover.
In the past ten years, Professor Liang Changhai and his group has done a lot of research work in the development of catalytic new materials such as metal silicides and metal aluminides in harsh reaction environments. He also published a series of international journals in the fields of catalytic science and chemical engineering. Systematic research results concerning the acid-resistant catalysts were published as follows. J. Catal., 2019, 369, 363; ChemCatChem, 2017, 9, 1337; ChemCatChem, 2015, 7, 978; J. Phys. Chem. C, 2015, 119, 29052; Catal. Today, 2015, 246,176; Catal. Sci. Technol., 2014, 4, 53(Inside Cover); J. Mater. Chem. C, 2014, 2, 5292(Back Cover); J. Phys. Chem. C, 2012, 116, 24968; Ind. Eng. Chem. Res., 2012, 51, 3604; J. Mater. Chem., 2012, 22, 609; Phys. Chem. Chem. Phys., 2011, 13, 9432; J. Phys. Chem. C, 2010, 114, 16525; J. Phys. Chem. C, 2010, 114, 3962; Chem. Commun., 2009, 2047.

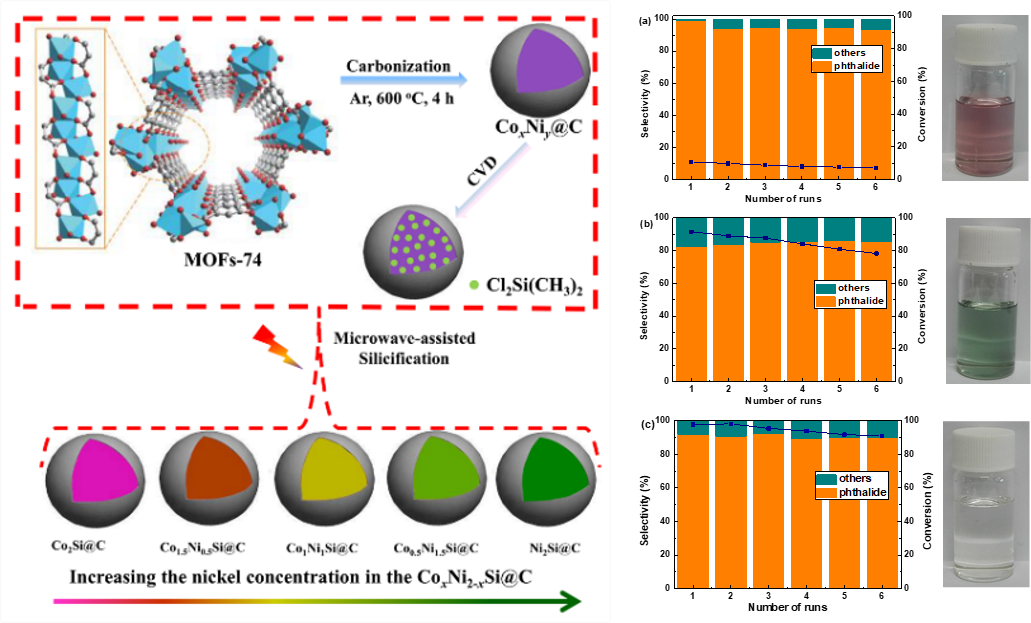
The above work was supported by the National Natural Science Foundation and the Special Research Funding of Dalian University of Technology. This is also one of the articles presented by the Lab of Advanced Materials & Catalytic Engineering for the 70th Anniversary of Dalian University of Technology!
Full article link: http://dx.