Since the rapid consumption of fossil fuels leads to the energy crisis and a series of environmental problems, it is necessary to find efficient, clean and renewable clean fuels, such as hydrogen, to replace non-renewable fossil fuels. As a promising hydrogen production method with high product purity, the electrocatalytic water splitting reaction includes two half-reactions: hydrogen evolution reaction (HER) at the cathode and oxygen evolution reaction (OER) at the anode. However, only under the condition of large overpotential can the high hydrogen production efficiency be achieved, which leads to low energy conversion efficiency and high hydrogen production cost. The development of efficient water splitting catalysts (especially bifunctional catalysts) can effectively reduce the overpotential and improve the energy conversion efficiency. Recently, a team of researchers led by Liang Changhai, a professor from School of Chemical Engineering, DUT, conducted a series of studies on the subject and produced some innovative results, which were published in ChemElectroChem, Electrochimica Acta, and ACS Applied Materials & Interfaces.
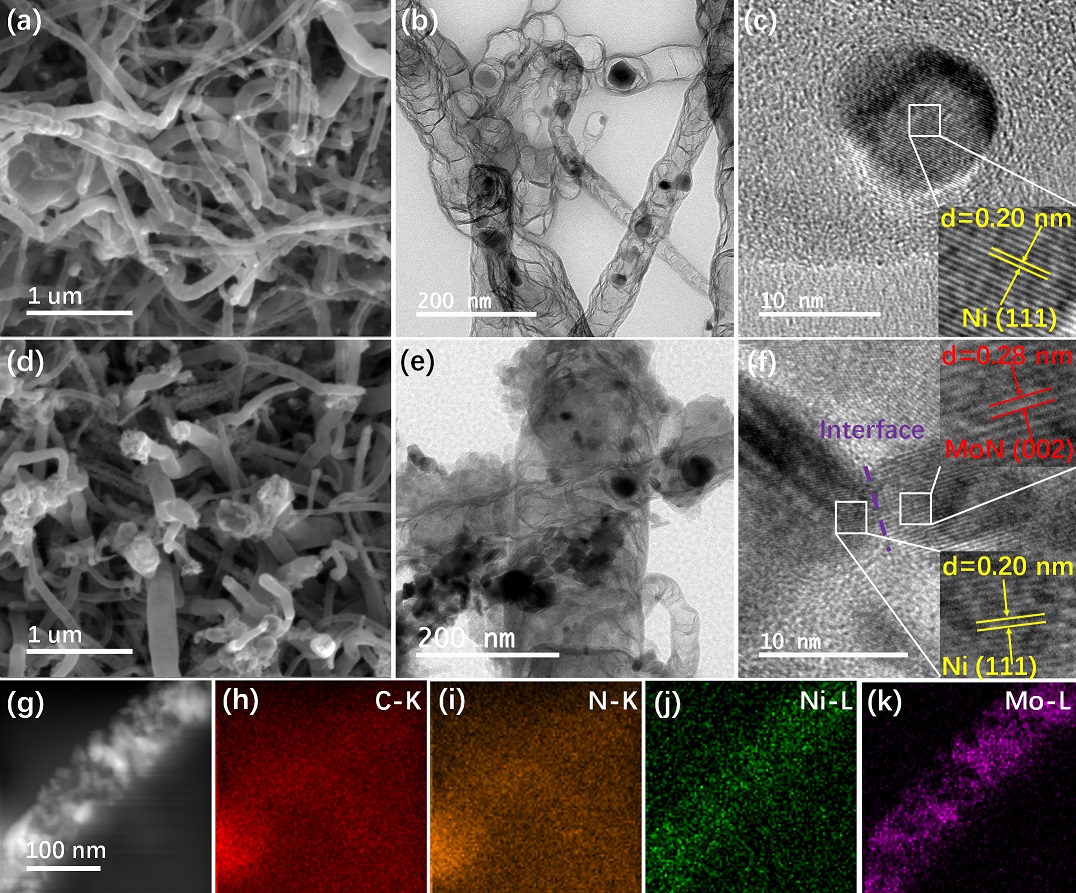
(1) Recently, they found that molybdenum nitride has been widely used as a catalyst for hydrogen evolution due to its catalytic properties similar to those of noble metals (VIII). However, it is still a challenge to improve the catalytic activity and stability of molybdenum nitride. A practical solution is to integrate molybdenum nitride with other functional materials to improve its catalytic performance. Ni-based materials are effective catalysts for OER. The composite of molybdenum nitride and Ni-based materials may improve the activity of the catalyst towards HER and OER. Besides, nano-sized catalysts tend to aggregate and reduce their catalytic activity during stability testing, which can be solved by anchoring or encapsulating them in carbon materials. Based on this assumption, the research team reported a new method for the synthesis of nitrogen-doped carbon nanotubes with Ni/MoN heterostructure on the surface of carbon cloth. Physical characterization and electrochemical test results show that the obtained catalyst has excellent HER, OER and overall water splitting (OWS) performance. This work provides a new strategy to construct a large surface area three-dimensional heterogeneous structure of the water decomposition catalyst. The work was published in the ChemElectroChem 2020,7,745-752 entitled “N-doped Carbon Nanotubes Encapsulating Ni/MoN Heterostructures Grown on Carbon Cloth for Overall Water Splitting”.
(2) Then, the research team found thatNi-Cobimetallic sulfides have shown excellent HER properties, but are oxidized at the oxygen evolution anode potential and becomes unstable as a result of sulfur atoms removement from the catalyst. LDH is a kind of OER catalyst with high activity and stability, but its weak conductivity limits its activity. Therefore, when Ni-Co sulfides and LDH are combined, the high conductivity of the former and the high stability of later can be used to compensate their shortcomings to finally achieve high activity towards water splitting. Since it has been reported that Mn can enhance the intrinsic activity of OER by adjusting the electronic structure of FeOOH, NiMn LDH may be a good substitute for the widely used NiFe LDH targeting at higher OER activity. In addition, the electrode can effectively contact the electrolyte as the reactant, which can accelerate the mass transfer and improve the catalytic activity. Based on the above strategies, a hierarchicalCoNi2S4@NiMn LDHheterostructure nanowire array was loaded onto a super hydrophilic carbon cloth. The heterostructures of theCoNi2S4@NiMn LDH not only combine the excellent intrinsic activities of theCoNi2S4nucleus and theNiMn LDHshell, but also compensate their shortcomings and enhance the conductivity and stability. The catalyst shows excellent activity and stability for HER, OER and OWS. For example, when it is used as both cathode and anode in the OWS, achieving current density of 10,50,100 and 200mA cm–2requires only 1.502,1.642,1.691 and 1.748 V of applied cell voltage, respectively. Such high activity is competitive among most non-noble metal catalysts at present. The work was published in the Electrochimica Acta (), entitled “Hierarchical CoNi2S4@NiMn-layered double hydroxide heterostructure nanoarrays on superhydrophilic carbon cloth for enhanced overall water splitting”.

(3) Based on the previous work, the research team found that the composite catalysts with heterogeneous structures showed stronger catalytic activity than each single component, which is a so-called “1 + 1 > 2” synergistic effect. NiCoPis a known excellent HER catalyst with high conductivity. Moreover,NiMn LDHhas shown excellent OER activity and stability. In this context, the research team has designed and synthesized a three dimensional heterostructural water splitting catalyst of NiCoP@NiMn LDHArray, which is supported on nickel foam. As a bifunctional catalyst for OWS, the catalyst has excellent activity: for OER, the current density of 100, 300 and 600 mA cm-2requiresoverpotential of 293, 315 and 327 mV, respectively; for HER, it takes only 116,130 and 136 mV of overpotential to reach the current density of 100,200 and 300 mAcm-2, respectively; For OWS, it requires only 1.519, 1.642, 1.671 and 1.687 V applied cell voltage to achieve current density of 10, 100, 200 and 300 mA cm-2, respectively. This excellent activity is superior to the majority of non-precious metal catalysts. Furthermore, for the OWS reaction, the catalyst remains stable for 50h at the current density of100 mA cm-2without an obvious decrease in activity. Such high efficiency can be attributed to the following three points: (a) The heterostructures existing between a NiCoP andNiMn LDH not only combine their respective high intrinsic activities, but also produce more active sites and enhance their intrinsic activities through interface effects; (b) The high conductivity of NiCoPenables a fast electron transport channel to enhance the conductivity of the electrode while the nickel foam with three-dimensional network structure can be used as a carrier to expose more active sites and accelerate mass transfer. The work was published in the Journal of ACS Applied Materials & Interfaces 2020, 12, 4385-4395 (), and featured as a cover story entitled“Three-Dimensional Heterostructured NiCoP@NiMn-Layered Double Hydroxide Arrays Supported on Ni Foam as a Bifunctional Electrocatalyst for Overall Water Splitting”.

This work was supported by the National Natural Science Foundation of China (21373038 and 21703028), the China Postdoctoral Science Foundation (2018M630290) and the Fundamental Research Funds for the Central Universities (DUT18RC(4) 042 and DUT16RC(3)053). The first author is Wang Pan, a 2016 doctoral student from School of Chemical, DUT. The corresponding author is Professor Liang, Changhai from School of Chemical Engineering, DUT. At present, the main research areas of Professor Liang’s team include heterogeneous catalytic synthesis of fine chemicals; efficient and clean conversion of unconventional resources to fuels and chemicals; environmental catalysis (catalytic combustion of VOC, co-denitrification and mercury removal);electrocatalyticenergy storage and conversion.